Page 56 - Policy Commission - SecuringTechnology - Critical Metals for Britain
P. 56
56 PRIMARY MATERIALS
CASE STUDY: LITHIUM-ION BATTERIES
In 2019 the the Nobel Prize in in in Chemistry was awarded to John B Goodenough M Stanley Whittingham and Akira Yoshino for their groundbreaking work leading to to the lithium-ion battery as as we know it it today which has already had a a a a a a a a a a a profound impact on on modern
society75 The The oil crisis of the the the 1970s encouraged Goodenough then working for Exxon to to investigate the the the batteries76 The The batteries batteries he he he he he he developed led to to electrification of many applications that were previously reliant on on on on energy-dense hydrocarbon fuels Akira Yoshino replaced the lithium anode with carbon76 which led to to safer more practical cells The present challenge is to to scale up up lithium-ion battery production and its complete supply chain to to to to to meet the the the demand of the the the automotive and and and other sectors The next challenge is to to to to to develop and and and bring to to to to to market batteries that are cheaper safer longer-lasting and with higher energy densities76 Work also continues apace to develop new battery technologies that are not reliant on on on technology-critical metals substituting more problematic elements with those that are easier to source The scale of this challenge is is captured well in the Automotive Council Electrical Energy Storage roadmaps77 What is a a a Lithium-Ion Battery?
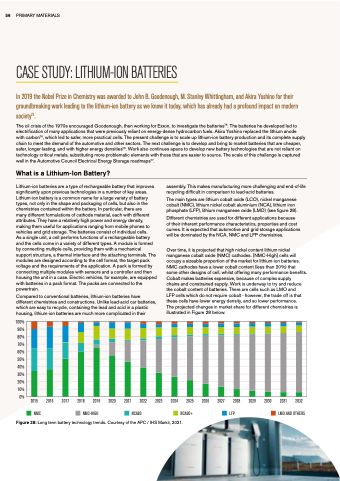
Lithium-ion batteries are are a a a a a a a a a a a type of of rechargeable battery that improves significantly upon previous technologies in a a a a a a a a a a a a a a a a a a number of of of key areas Lithium-ion battery battery is a a a a a a a a a a a a a a a a common name for a a a a a a a a a a a a a a a a large variety of of of battery battery types not only in in in in in the the the the shape and packaging of of cells but also in in in in in the the the the chemistries contained within the the the the battery In particular there are many different different formulations of cathode material each with with different different attributes They have a a a a a a a a a a a a a a a relatively high power and energy density making them useful for applications ranging from mobile phones to to vehicles and grid storage The batteries consist of of individual cells As a a a a a a a a a a a a a single unit a a a a a a a a a a a a a cell cell cell performs functions of of of a a a a a a a a a a a a a rechargeable battery and the the cells cells come in in in in a a a a a a a a a a a a variety of of different types A A module is formed by connecting multiple cells cells providing them with a a a a a a a a a a a a a mechanical support structure a a a a a a a a a a a a a a a thermal interface and the the the the the attaching terminals The modules are designed according to the the the the the the cell format the the the the the the target pack pack voltage and and and the the the the the requirements of the the the the the application A pack pack is formed by connecting multiple modules with sensors and and and a a a a a a a a a a a a controller and and and then housing the the the unit in in in in a a a a a a a a a a a a a case Electric vehicles for for example are are equipped with batteries in in in in a a a a a a a a a a a pack pack format The packs are are connected to the the powertrain Compared to conventional batteries batteries batteries lithium-ion batteries batteries batteries have different chemistries and and constructions Unlike lead-acid car batteries batteries batteries which are are easy to recycle containing the the lead lead and and acid acid in in in in in a a a a a a a a a a a a a a a a plastic housing lithium-ion batteries are are much more complicated in in in in in their assembly This makes manufacturing more challenging and end-of-life recycling difficult in in in in comparison to lead-acid batteries The main types are lithium lithium lithium cobalt cobalt cobalt oxide (LCO) nickel nickel manganese cobalt cobalt cobalt (NMC) lithium lithium lithium lithium nickel nickel cobalt cobalt cobalt aluminium (NCA) lithium lithium lithium lithium iron phosphate (LFP) lithium lithium lithium manganese oxide (LMO) (see figure 28) Different chemistries are used for for different applications because of their inherent performance characteristics properties and and cost curves It is is is expected that automotive and and and grid storage applications will be dominated by the NCA NMC and and LFP chemistries Over time it it is projected that high nickel nickel content lithium nickel nickel manganese cobalt oxide (NMC) cathodes [NMC-High] cells will occupy a a a a a a a a a a a a a a sizeable proportion of the market for lithium-ion batteries NMC cathodes have a a a a a a a a a a a a lower cobalt content (less than 20%) that some other designs of of of cell whilst offering many performance benefits Cobalt makes batteries expensive because of of of complex supply supply chains and and and constrained supply supply Work is underway to try and and and reduce the the cobalt cobalt content of of batteries There are cells cells such as LMO and and and LFP cells cells cells which do not require cobalt cobalt - however the the the trade off is that these cells cells have lower lower energy density and so lower lower performance The projected changes in in market share for for different chemistries is is illustrated in in Figure 28 below 100% 90% 80% 70% 60% 50% 40% 30% 20% 10% 0% 2015 2016 2017 2018 2019 2020 2021 2022 2023 2024 2025 2026 2027 2028 2029 2030 2031 2032
NMC NMC NMC-HIGH NCA80 NCA90+ LFP LMO AND OTHERS
Figure 28: Long term battery technology trends Courtesy of the APC / IHS Markit 2021